Macrophage-mediated diseases indications
Allocetra™ pipeline building momentum:
Strong phase 2 progress with next steps in sight
Indication:
Pre-clinical
Phase I/II
Phase II
Phase III
ENX-CL-05-001
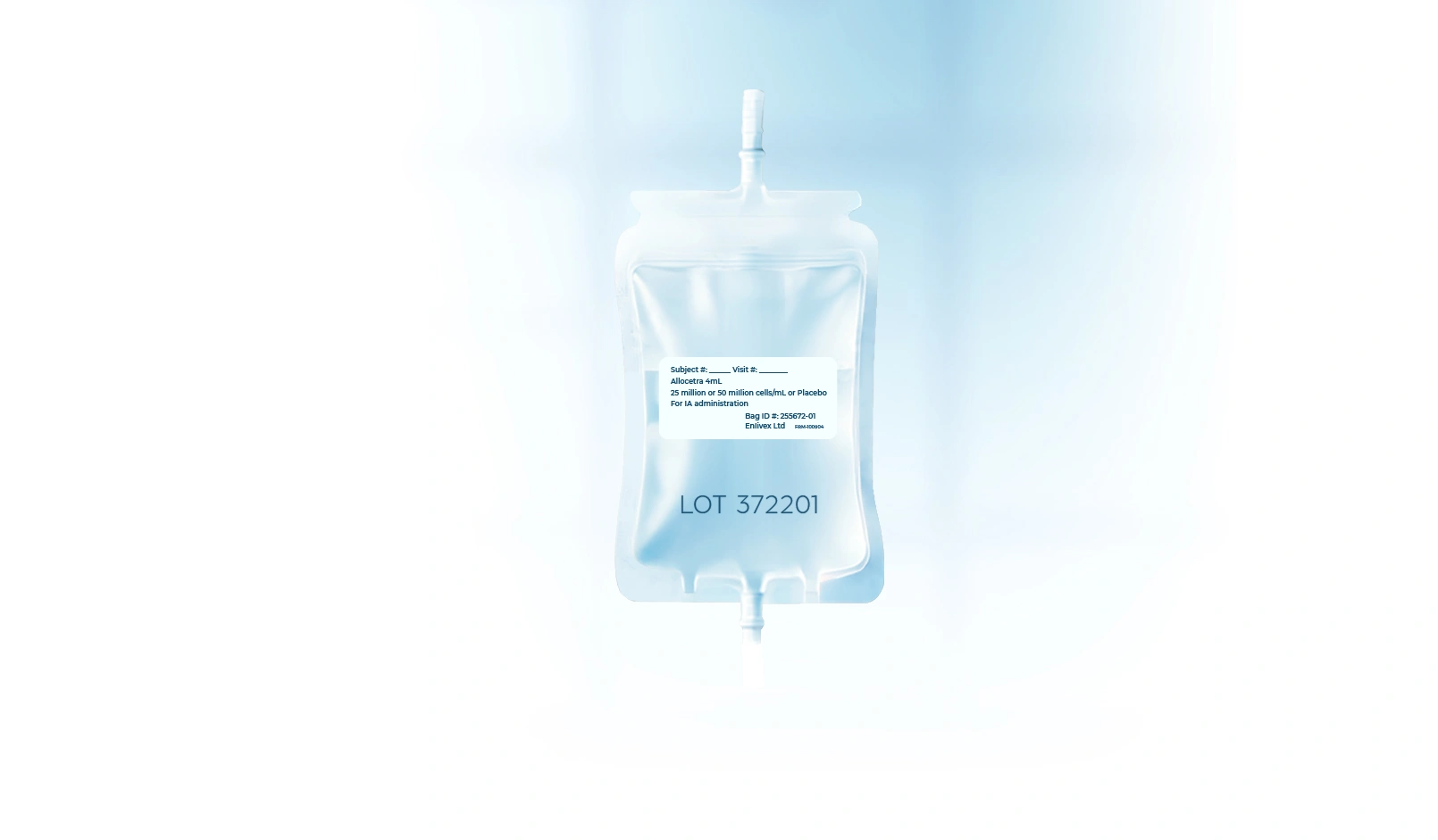
Moderate knee
osteoarthritis
A double blind, randomized, multi-center study to evaluate the safety and efficacy of intra-articular administration of Allocetra™ compared to placebo in patients with symptomatic knee osteoarthritis.
View program1400-24-SMC
Temporomandibular joint
osteoarthritis
A single center, Investigator-initiated study to evaluate the safety and initial efficacy of intra-articular administration of Allocetra™ to patients with Temporomandibular Joint Osteoarthritis (TMJ-OA).
View programENX-CL-02-002
Organ failure associated
with Sepsis
A phase 2, multi-center, randomized, placebo-controlled, dose-finding study evaluating efficacy, safety and tolerability of different doses and regimens of Allocetra™ for the treatment of organ failure in adult sepsis patients.
View program